Product Details
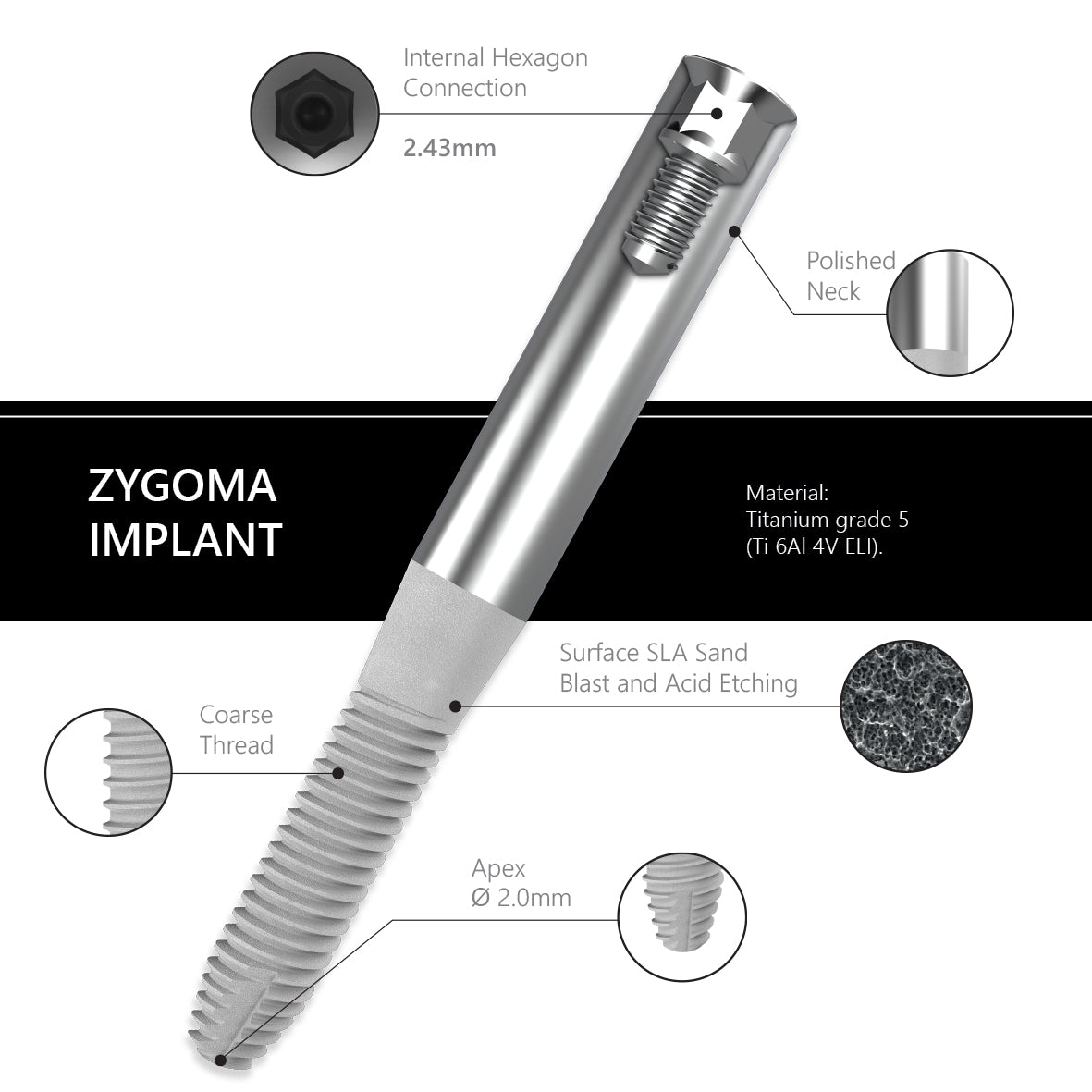
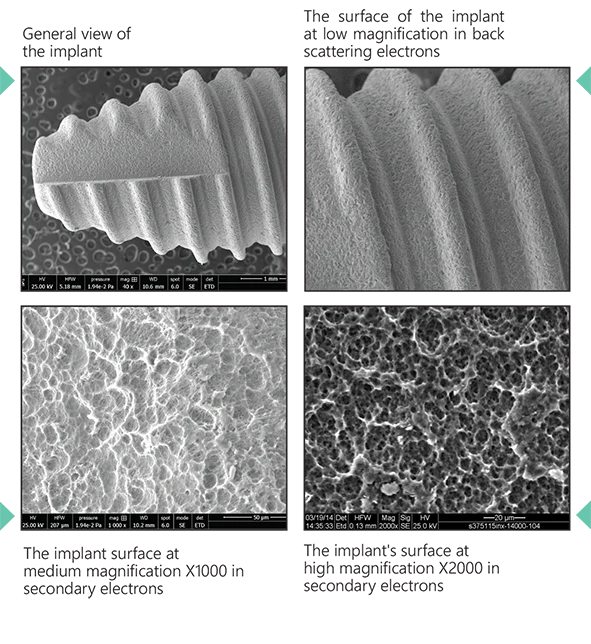
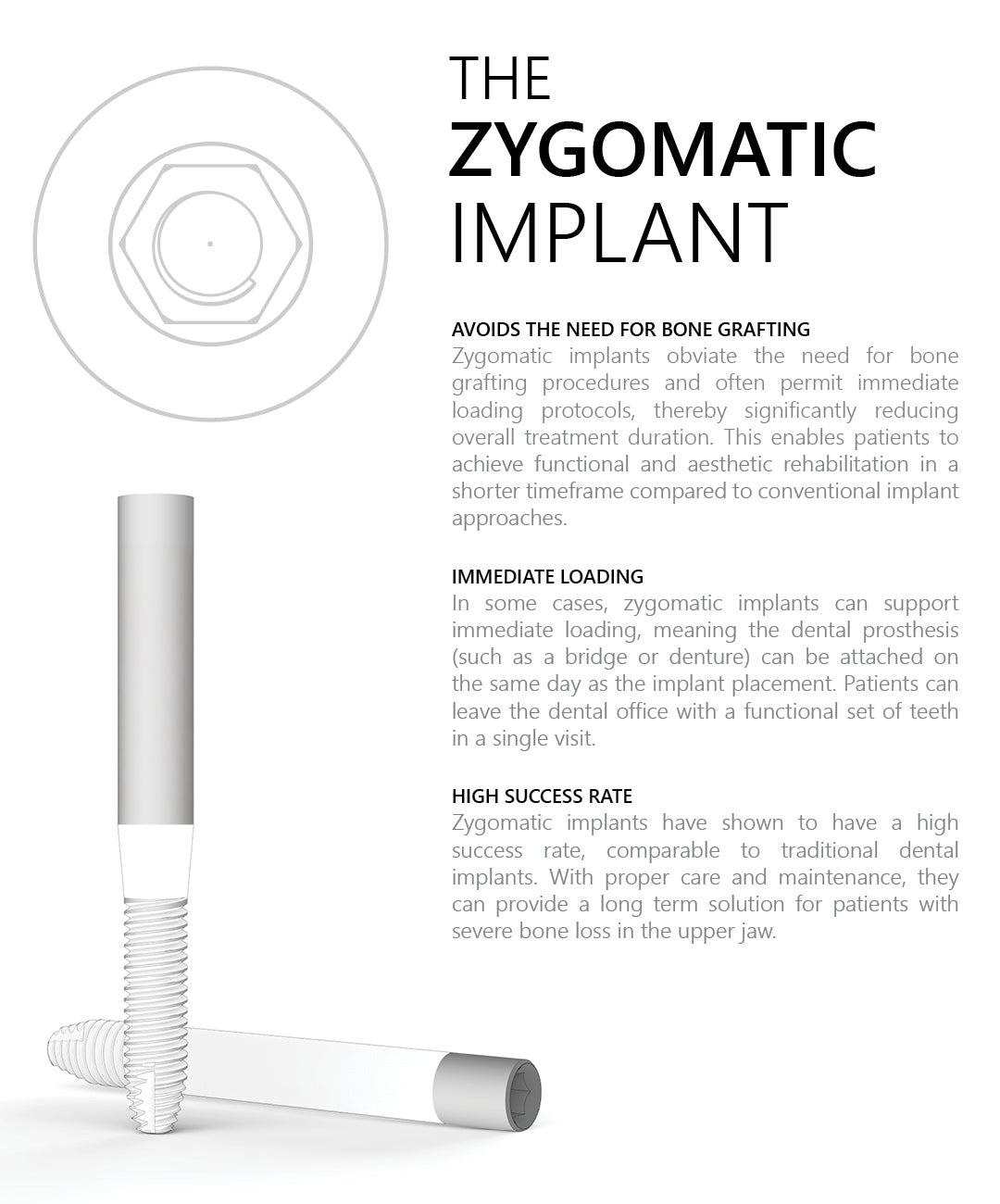
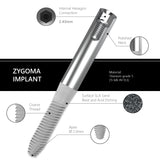
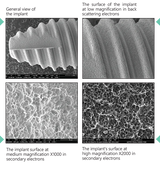
The Surcam Zygomatic Implant is designed for patients with severe maxillary atrophy who are not candidates for grafting or sinus augmentation. By anchoring in the zygomatic (malar) bone, it provides a dense, stable foundation for fixed full-arch restorations. The implant’s cylindrical core with dual/multiple thread pattern promotes controlled advancement, while continuous crestal micro-threads support blood flow and cervical stress modulation. An anchoring apex aids trajectory control through the zygomatic pathway. The SLA surface (dry alumina blasting + double acid etching) supports predictable osseointegration.
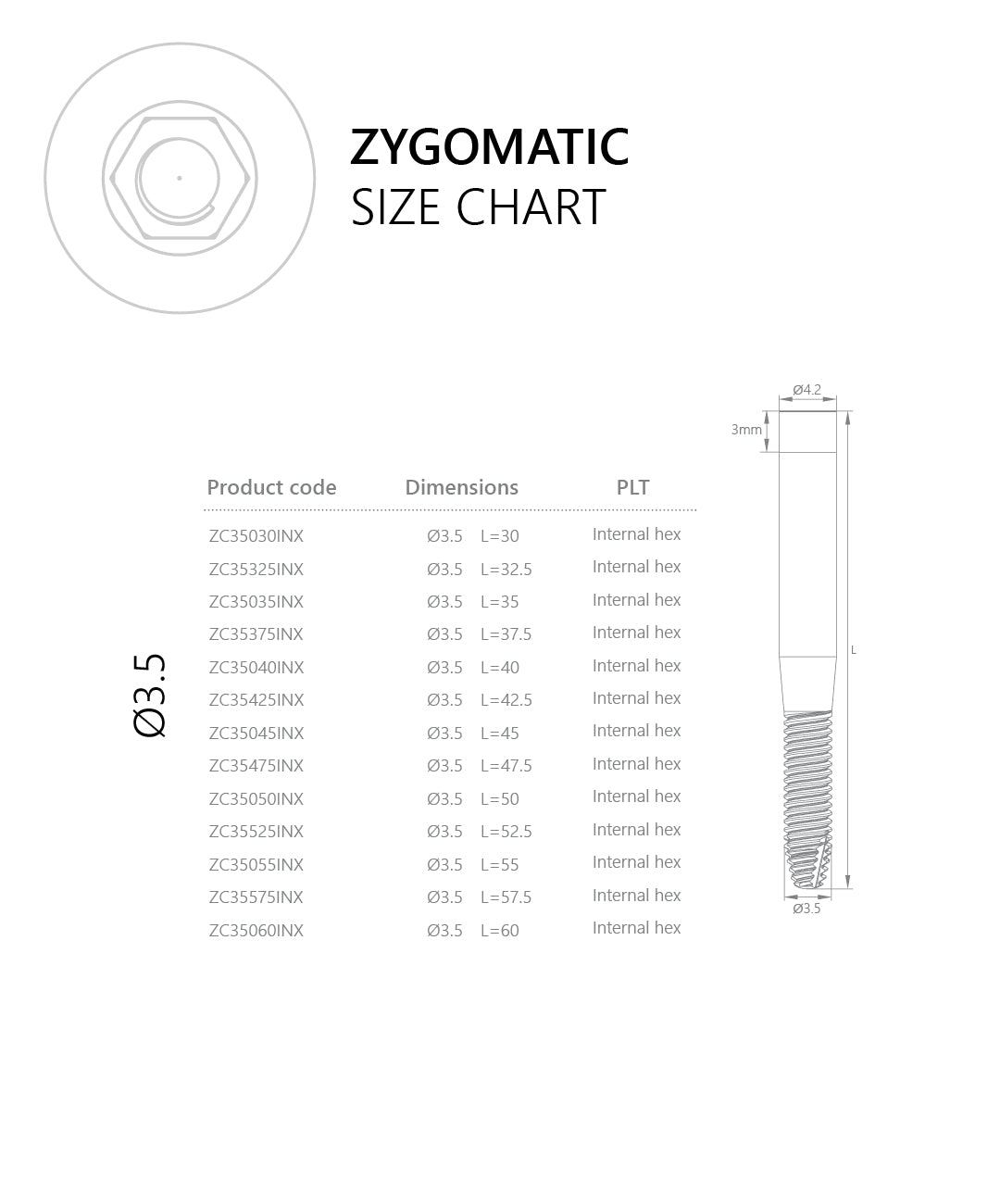
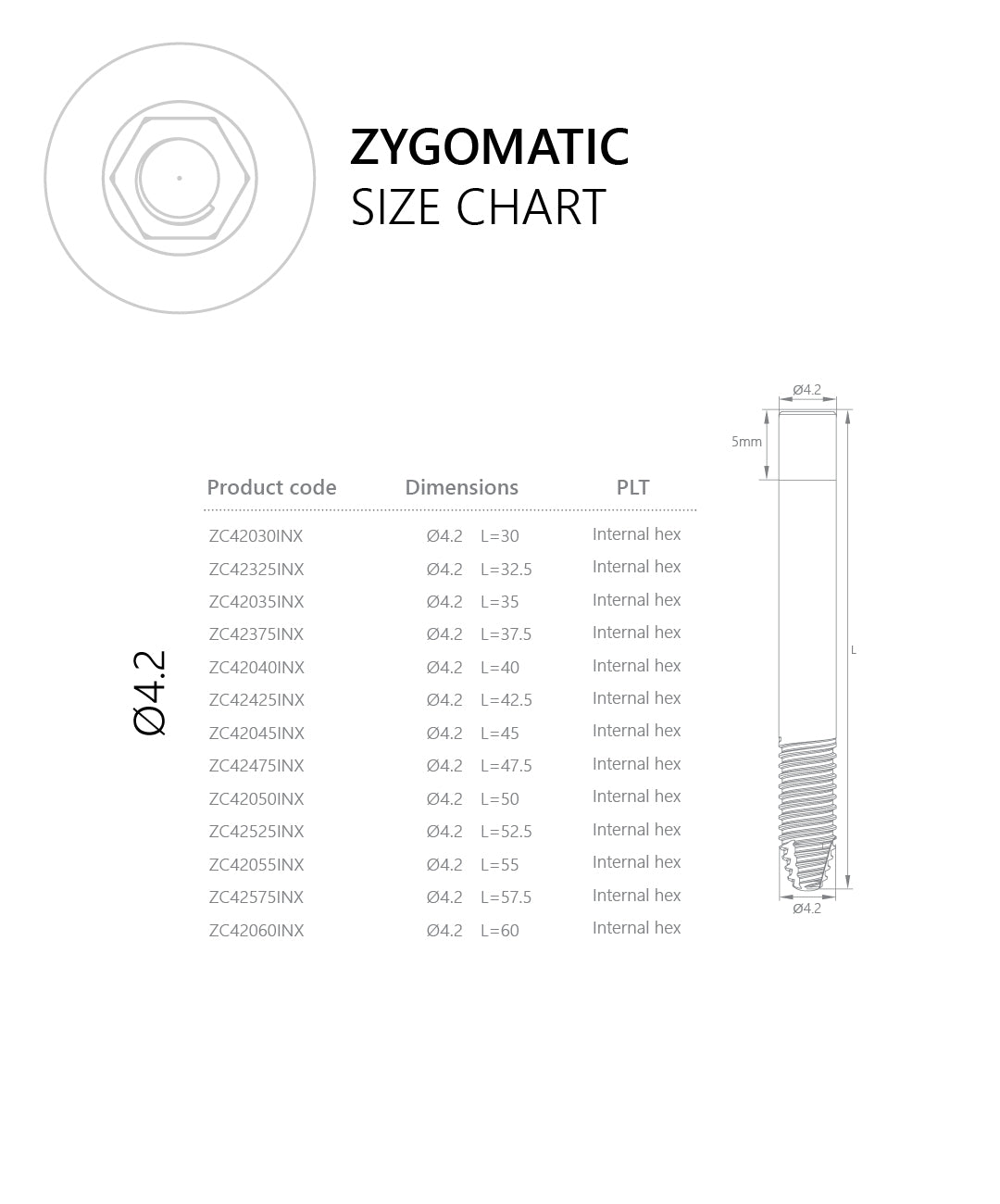
Connection / Platform: Internal Hex 2.43 mm (verify interface/fit). Immediate loading may be considered case-dependent when stability and occlusion meet IFU criteria. Advanced planning (CBCT, guided trajectory) and specialist training are essential.
FAQs
Is this implant indicated for all atrophic maxilla cases?
No. It’s intended for severe maxillary atrophy where conventional fixtures or grafting are not feasible. Final indication is case-dependent following CBCT and surgical planning per IFU.
Can it be used for immediate loading?
Potentially. Immediate/early loading may be considered only when primary stability (e.g., torque/ISQ) and occlusal control meet IFU criteria for full-arch rehabilitation.
What planning is required before placement?
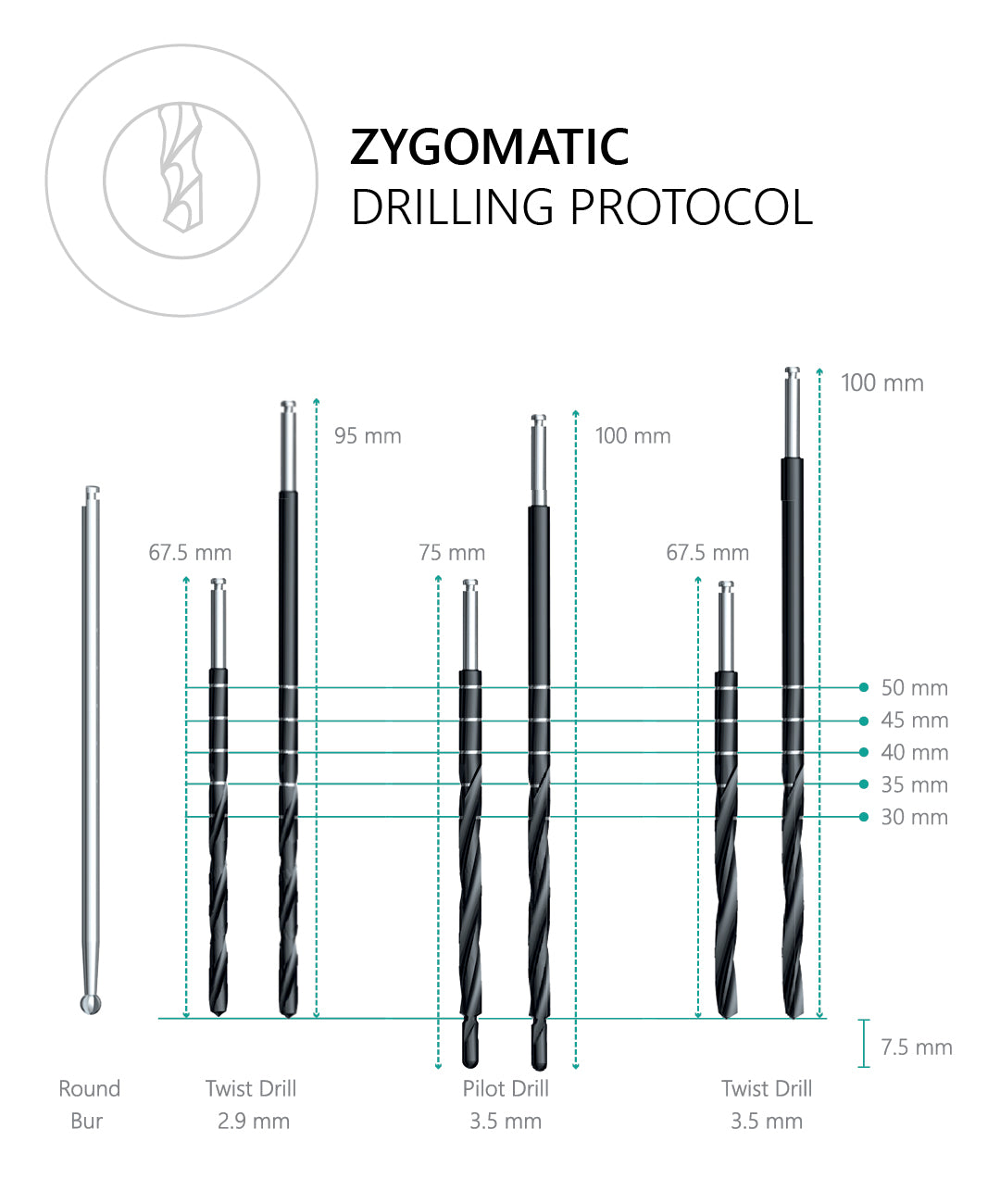
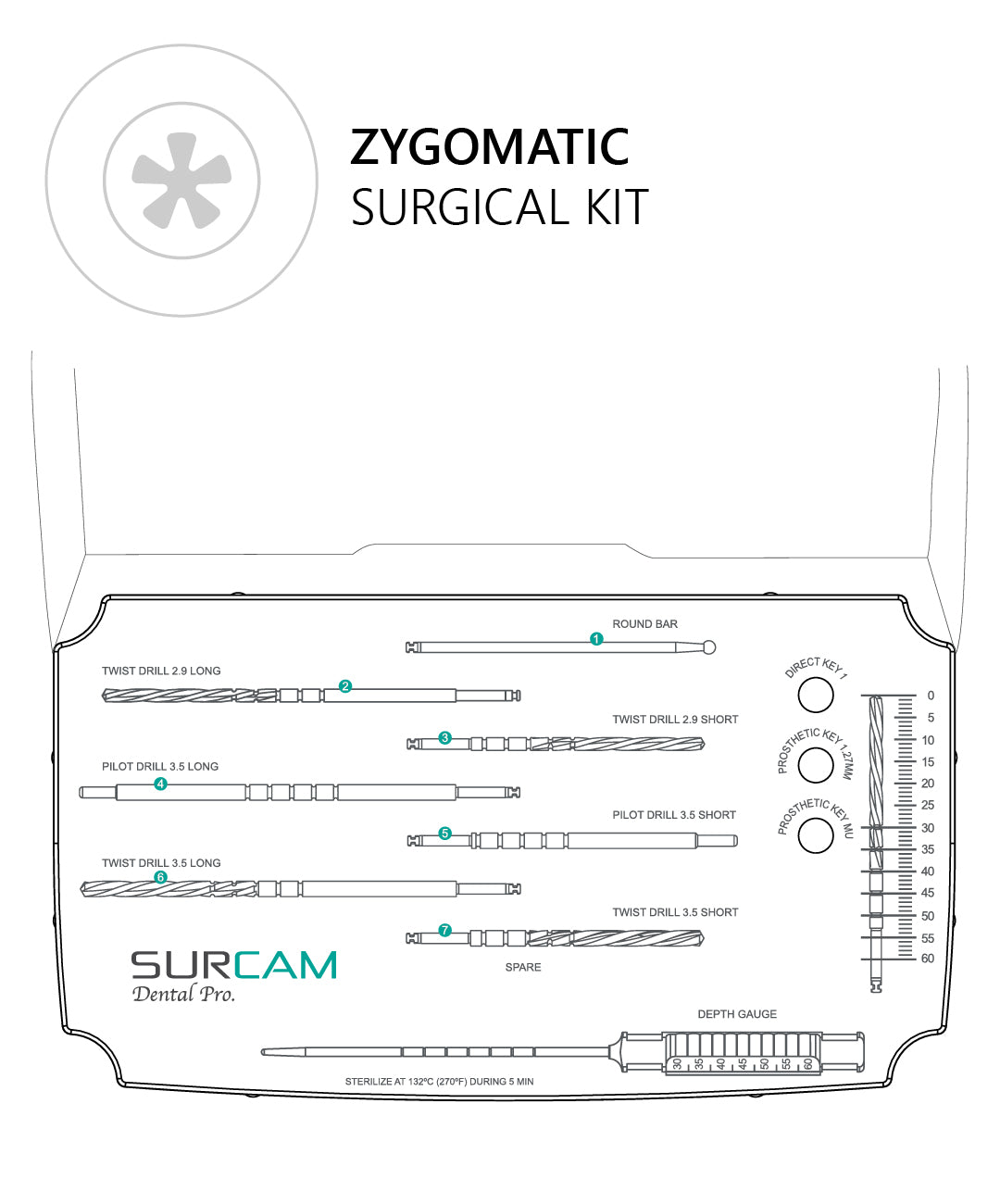
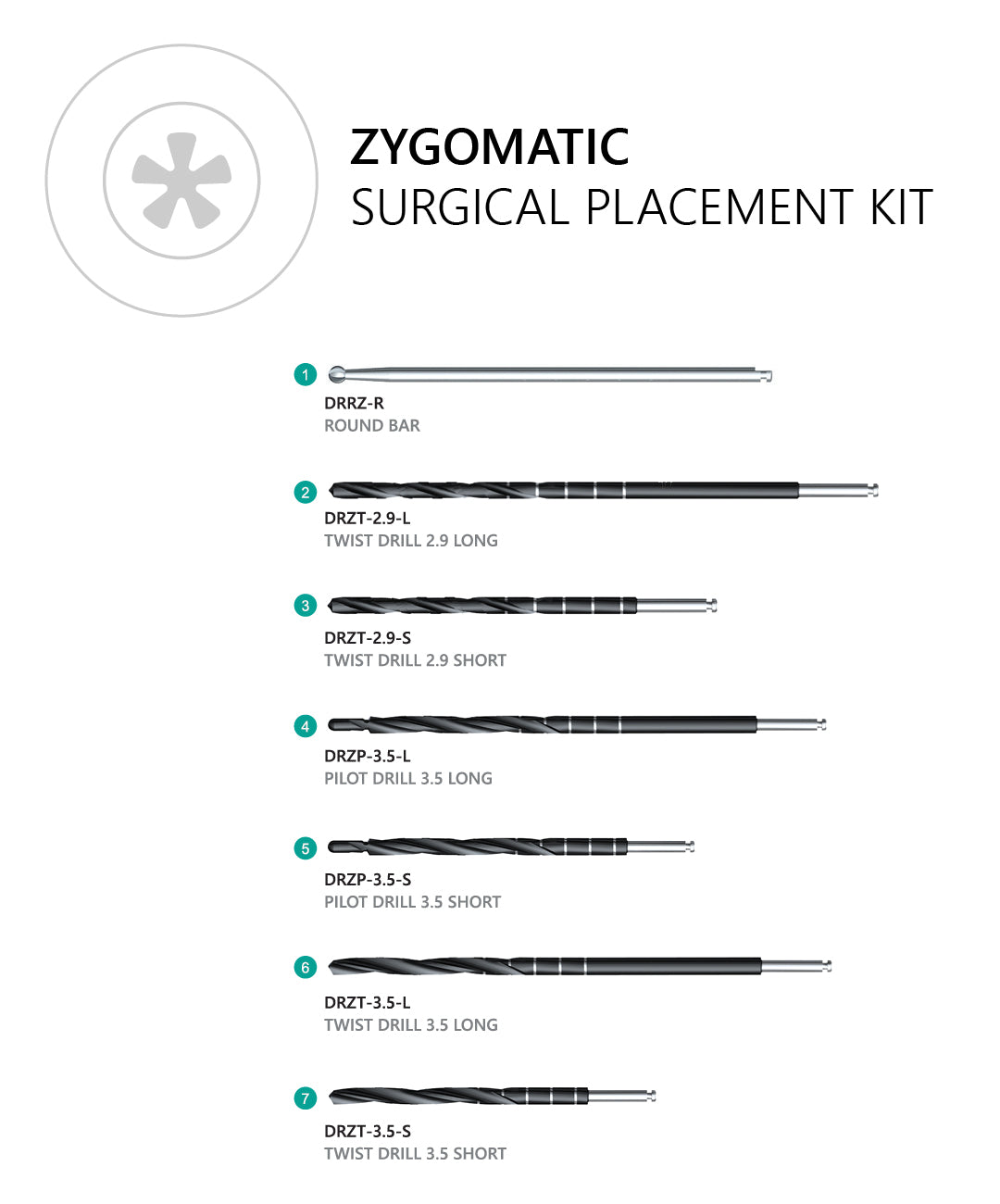
A CBCT-based plan with assessment of zygomatic trajectory, sinus anatomy, and prosthetic space is essential. Consider guided surgery and specialist training.
Which restorative components are compatible?
Use Internal Hex 2.43 mm compatible components (e.g., multi-unit abutments/frameworks) matched to the prosthetic plan. Verify platform code and indexing before use.
What surgical precautions apply in the zygomatic pathway?
Follow IFU and surgical protocol: control angulation, irrigation, and trajectory; avoid over-torque; respect sinus/soft-tissue structures. Post-op monitoring is recommended.